Axitinib对转移性肾细胞瘤具有活性思维导图
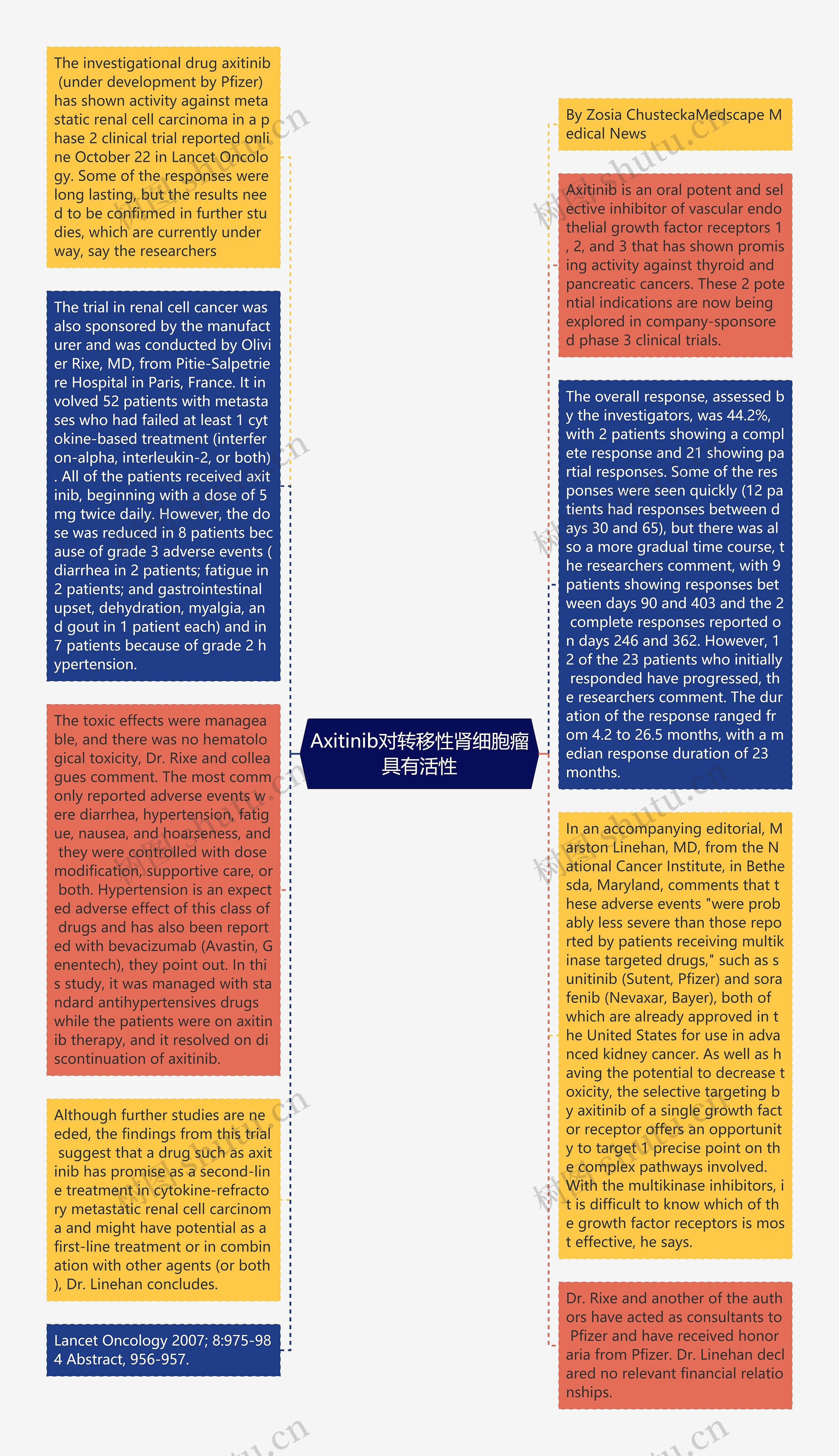
正在研究中的药物axitinib(由辉瑞药厂研发中),在第二期临床研究中显示对于转移性肾细胞瘤具有活性,项研究发表于10月22日的Lacent肿瘤学期刊上;研究者表示,某些反应是可以持续很久的,但是该研究结果需要在后续研究中获得证实,这些研究目正在进行中Axitinib是一种口服效价高,且对于血管内皮生长因子受体第一、二、三型具有活性的选择性抑制剂,且已经被证实可以有效对抗甲状腺与胰脏癌;这两具有潜力的适应症目前正在一项由厂商赞助的第三期临床试验中进行研究。这项由制造商赞助、针对肾细胞癌的研究,是由法国巴
树图思维导图提供 Axitinib对转移性肾细胞瘤具有活性 在线思维导图免费制作,点击“编辑”按钮,可对 Axitinib对转移性肾细胞瘤具有活性 进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:b92de36e705c06d3d27e3b211bdd2396
思维导图大纲
Axitinib对转移性肾细胞瘤具有活性思维导图模板大纲
By Zosia ChusteckaMedscape Medical News
The investigational drug axitinib (under development by Pfizer) has shown activity against metastatic renal cell carcinoma in a phase 2 clinical trial reported online October 22 in Lancet Oncology. Some of the responses were long lasting, but the results need to be confirmed in further studies, which are currently under way, say the researchers
Axitinib is an oral potent and selective inhibitor of vascular endothelial growth factor receptors 1, 2, and 3 that has shown promising activity against thyroid and pancreatic cancers. These 2 potential indications are now being explored in company-sponsored phase 3 clinical trials.
The trial in renal cell cancer was also sponsored by the manufacturer and was conducted by Olivier Rixe, MD, from Pitie-Salpetriere Hospital in Paris, France. It involved 52 patients with metastases who had failed at least 1 cytokine-based treatment (interferon-alpha, interleukin-2, or both). All of the patients received axitinib, beginning with a dose of 5 mg twice daily. However, the dose was reduced in 8 patients because of grade 3 adverse events (diarrhea in 2 patients; fatigue in 2 patients; and gastrointestinal upset, dehydration, myalgia, and gout in 1 patient each) and in 7 patients because of grade 2 hypertension.
The overall response, assessed by the investigators, was 44.2%, with 2 patients showing a complete response and 21 showing partial responses. Some of the responses were seen quickly (12 patients had responses between days 30 and 65), but there was also a more gradual time course, the researchers comment, with 9 patients showing responses between days 90 and 403 and the 2 complete responses reported on days 246 and 362. However, 12 of the 23 patients who initially responded have progressed, the researchers comment. The duration of the response ranged from 4.2 to 26.5 months, with a median response duration of 23 months.
The toxic effects were manageable, and there was no hematological toxicity, Dr. Rixe and colleagues comment. The most commonly reported adverse events were diarrhea, hypertension, fatigue, nausea, and hoarseness, and they were controlled with dose modification, supportive care, or both. Hypertension is an expected adverse effect of this class of drugs and has also been reported with bevacizumab (Avastin, Genentech), they point out. In this study, it was managed with standard antihypertensives drugs while the patients were on axitinib therapy, and it resolved on discontinuation of axitinib.
In an accompanying editorial, Marston Linehan, MD, from the National Cancer Institute, in Bethesda, Maryland, comments that these adverse events "were probably less severe than those reported by patients receiving multikinase targeted drugs," such as sunitinib (Sutent, Pfizer) and sorafenib (Nevaxar, Bayer), both of which are already approved in the United States for use in advanced kidney cancer. As well as having the potential to decrease toxicity, the selective targeting by axitinib of a single growth factor receptor offers an opportunity to target 1 precise point on the complex pathways involved. With the multikinase inhibitors, it is difficult to know which of the growth factor receptors is most effective, he says.
Although further studies are needed, the findings from this trial suggest that a drug such as axitinib has promise as a second-line treatment in cytokine-refractory metastatic renal cell carcinoma and might have potential as a first-line treatment or in combination with other agents (or both), Dr. Linehan concludes.
Dr. Rixe and another of the authors have acted as consultants to Pfizer and have received honoraria from Pfizer. Dr. Linehan declared no relevant financial relationships.
Lancet Oncology 2007; 8:975-984 Abstract, 956-957.
相关思维导图模板

树图思维导图提供 数学如何解决问题? 在线思维导图免费制作,点击“编辑”按钮,可对 数学如何解决问题? 进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:1b17bf503628837a34235fb7a84f5863
树图思维导图提供 SpringBootWeb请求响应 在线思维导图免费制作,点击“编辑”按钮,可对 SpringBootWeb请求响应 进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:1c6ee1ff958a0c7c2fabdf9e9f8d755e








 上海工商
上海工商